 _____ Publications
_____ Publications Graphical summary of our research group 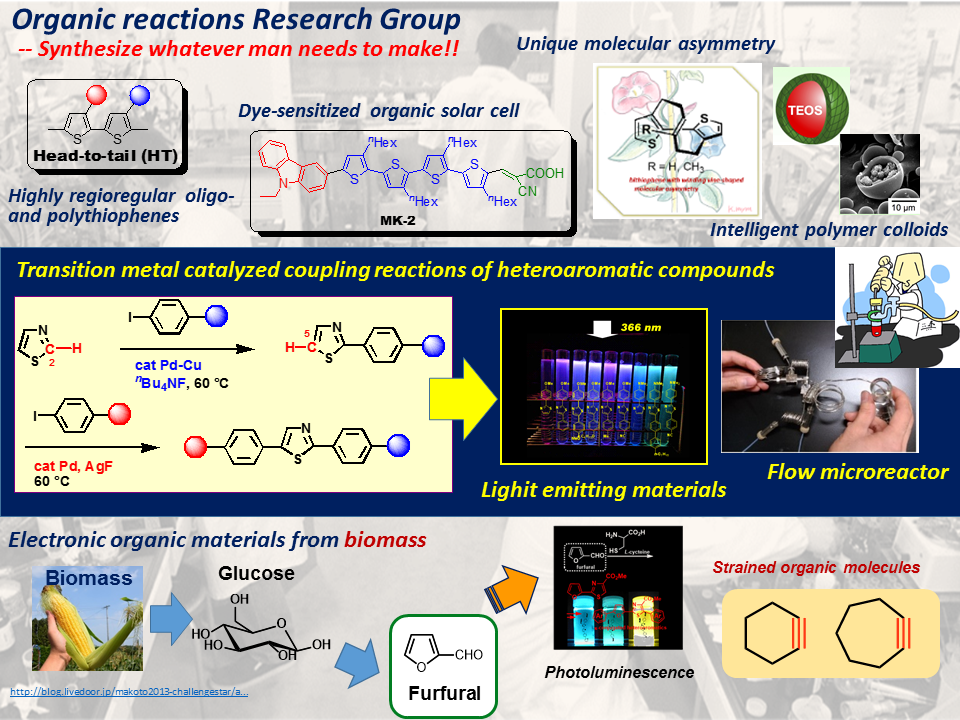 click to expand | The Organic Reactions Research Group is interested in the development of new synthetic reactions that are potentially available for materials science, pharmaceutical science, etc. Heteroaromatic compounds such as thiophene and thiazole derivatives undergoes coupling reactions at the C-H bond to form carbon-carbon bond. The reactions are successfully applied to form conjugated oligomers and polymers available as electronic materials. Winding-vine shaped heterobiaryls show molecular asymmetry, in which axial, helical, and facial chiralities are involved in a single molecule. Polymer colloids of unique structures are prepared in a facile manner. Biomass derived heteroaromatic furfural is also employed for the coupling reaction forming conjugated molecules. Several strained organic small molecules are found to be prepared in a simple operation. |
・Transition metal catalysis
Activation of the CH bond of heteroaromatic compounds with a transition metal catalyst is performed. Coupling reaction of thus formed metal species with various organic electrophiles to form carbon-carbon and carbon-heteroatom bonds is developed. Application of these reactions toward advanced organic materials and biologically active molecules is our major concern.
Thiophenes & thiazoles as advanced materials 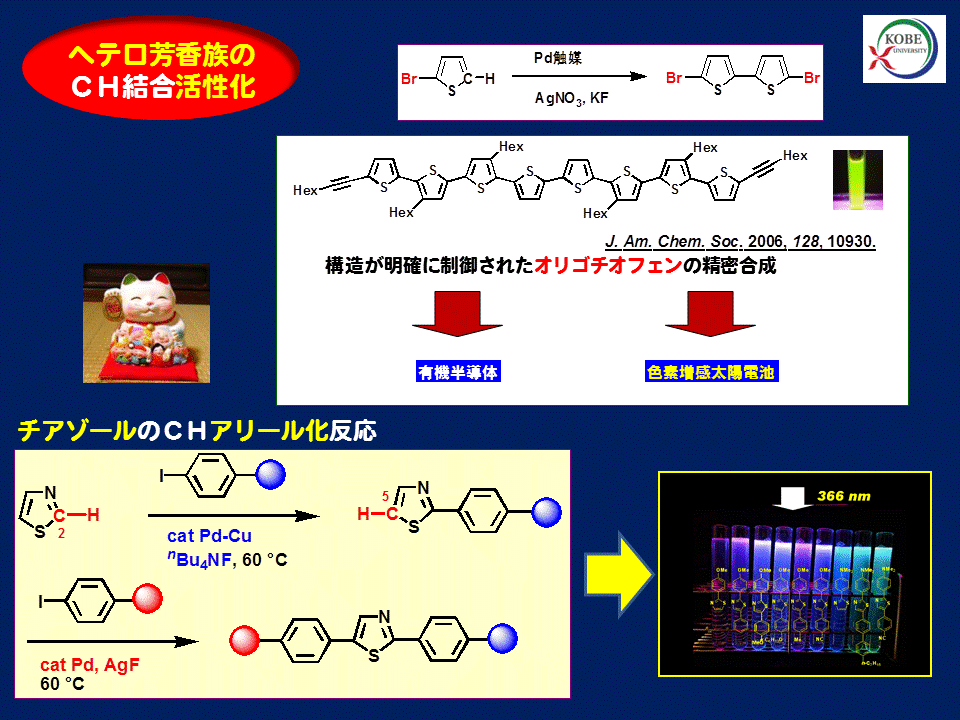 J. Am. Chem. Soc., 2003, 125, 1700 J. Am. Chem. Soc., 2004, 126 , 5074 J. Am. Chem. Soc. 2006, 128, 10930 J. Am. Chem. Soc. 2011, 133, 16734 | New synthetic methodology of polythiophene synthesis with C-H coupling polymerization Metalation at the 5-position of 2-chloro-3-hexylthiophene and 2-bromo-3-hexylthiophene with magnesium amide or the combination of catalytic secondary amine and Grignard reagent and following addition of a nickel catalyst induces polymerization affording highly regioregular head-to-tail-type poly(3-hexylthiophene). Design and synthesis of regioregular head-to-tail-type linear and branched oligothiophens Regioselective metalation of 3-hexylthiophene at the 5-position and following nickel-catalyzed coupling with halothiophene forms thiophene-thiophene bond. A variety of linear and branched oligothiophenes are synthesized highly efficiently. ここに空白を挿入Recent papers: |
・Deprotonative CH coupling leading to polythiophenes
Polythiophene synthesis via CH coupling reactions 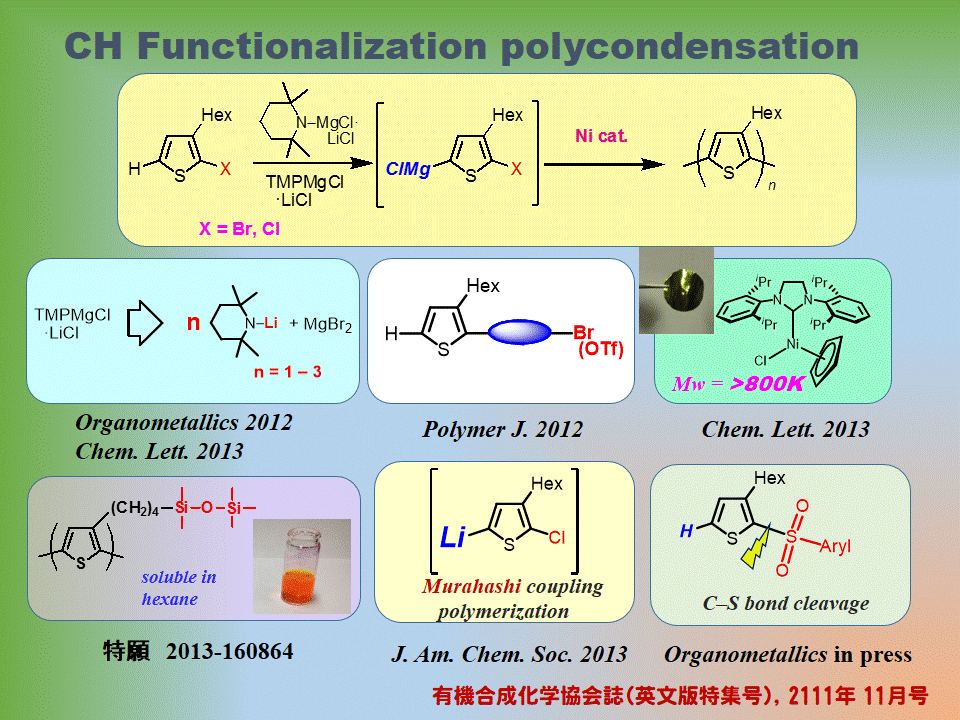 Chem. Lett. 2011, 40, 398 J. Am. Chem. Soc. 2011, 133, 9700 J. Am. Chem. Soc., 2013, 135, 12208 | Employment of bulky magnesium amide such as Knochel-Hauser base to the reaction of 2-halo-3-substituted thiophene undergoes deprotonation at the 5-position. Following addition of a nickel(II) catalyst induces polymerization to afford head-to-tail-type highly regioregular polythiophenes in a facile manner. 1. dehydrobrominative polymerization 2. Chlorothiophene is also available!! Combination of RMgX and catalytic R2NH can also be employed instead. 3. Synthesis of poly(thienylene arylene)s 4. Extremely high-molecular-weight polythiophene (Mw: higher then 800 KDa; Photo: Self-standing film of thus obtained polythiophene) 5. Combination of lithium amide and MgX2 (ratio:1-3) wil also be replaced with Knochel-Hauser base 6. Formation of thiophene-Li species: Murahashi coupling polymerization. 7. Cross-coupling polymerization via C-S bond cleavage. Review: J. Synth. Org. Chem. Jpn., 2011, 69 (11: special issue in English), 1202-1211. ☆ ☆ ☆ ☆ ☆ ☆ |
Macrocyclic olefin bearing axially chiral bisazole, Synthesis and Optical resolution Winding-vine like molecular asymmetry
Winding vine-shaped molecular asymmetry 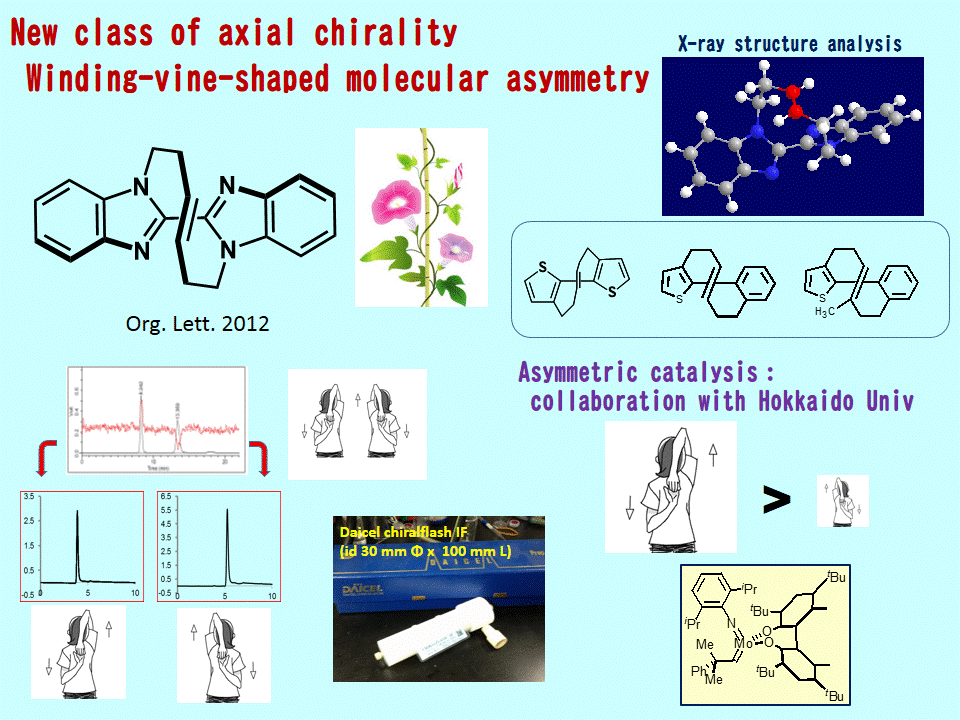 | Bisazole, which is prepared by homocoupling of imicazole derivatives bearing olefinic alkylene substituent on the nitrogen atom, gives E-selective macrocyclic olefin by Ring-closing metathesis with Ruthenium (Grubbs') catalyst. Formation of the macrocyclic structure is confirmed by X-ray analysis. This compound results in molecular asymmetry (axially chiral) and separation of the enantiomers can be separated by HPLC with chiral column. Org. Lett., 2012, 14 (10), pp 2476–2479 Angew. Chem. Int. Ed. 2015, 54 (16). 4927-4931 ☆ ☆ ☆ ☆ ☆ ☆ |
New preparative protocol for gold nanoparticles with organosilicon compound as a reducing agent
Kenta Kumazawa has God hands!
Kenta Kumazawa achieved Suzuki-Miyaura coupling on the surface of gold nanoparticle. Nobody in our group members has previously perfomed successfully. Sonogashira coupling could also be done by Kumazawa
Synthesis and performance of TPEN derivatives: Design of functional ligand
Design and synthesis of functional ligand that can chelate efficiently to soft metals. TPENs are well known to chelate minor actinides selectively and extract Am (americium) and Cm (Curium) from high-level-waste in the recycle of nuclear waste. Based on strong background of Organic Synthesis, this project supports development of innovative nuclear power source recycle system by persueing efficient synthesis of TPEN derivatives.
(休眠中)
Our researches on cover pages
 | ||
| TCI mail, 2012, No. 153 | ||
| Approach with CH coupling reactions ******************************************* | ****************************************** | ****************************************** |
_





コメントをかく